Fluorides are present throughout the world in the earth’s crust. That’s why they’re naturally found in everything — including the soil, rocks, plants and water all over the earth.
But did you know fluoride may hurt your health, including your bones? While still a controversial topic, especially when it comes to fluoride preventing tooth decay, I’m going to dive into the research so you can make an informed decision about fluoride.
But first, let’s differentiate between fluoride and fluorine – as you may have seen both terms used before.
Fluoride vs. Fluorine
Are you confused about the difference between fluoride and fluorine? You’re not the only one.
To answer this common chemistry question simply…
Fluorides are compounds that contain the element fluorine (F-). Fluorine is an ion (a molecule with a net electric charge due to the loss or gain of one or more electrons). In fluorine’s case, the net electric charge is a negative one, which makes fluorine an anion (a molecule with a negative charge). Bear with me as I get to how this is important for you…
Just like all other elements (which are also ions with either a negative charge, in which case they are called anions, or a positive charge, in which case, they’re called cations), fluorine is unstable by itself and does not appear alone. It combines with a stabilizing partner, which for fluorine will be a compound with a positive charge or cation. So, when we are talking about fluoride, we are actually talking about the chemical element fluorine in one of its stable fluoride forms.
Water fluoridation for instance, occurs when a fluorine-containing compound is added to drinking water. Common sources are sodium fluoride, sodium fluorosilicate or fluorosilicic acid, which are added to the water and dissociates, releasing its F- ion.
Fluorides used in toothpaste and other dental treatments are typically sodium fluoride or stannous fluoride.
In addition, fluorides are used in numerous industrial processes:
- Coal burning
- Oil refining
- Steel production
- Brick-making
- Phosphate fertilizer production (Yup, the fertilizers used in conventional farming. Another reason to eat organically grown foods!)
But what do fluorides mean for your health? And in particular…your bones?
Let’s find out…
Ever Present Fluoride: In Your Tap Water, Toothpaste and Harming Your Bones?
The primary sources of exposure to fluorides are our food and water, and fluoride-containing dental products, such as toothpaste.
Fluoride is found in higher concentrations in the southern United States and in all areas where the waters are soft, alkaline, and calcium-deficient.
Fluoride compounds that occur naturally in drinking water are almost totally bioavailable – at about 90%! That is, the fluorine they contain is virtually all absorbed from the gastrointestinal tract and enters our circulatory system.
The addition of extra fluoride to community drinking water, via its fluoridation in an effort to prevent cavities, has resulted in a significant increase in dental fluorosis– a disturbance in the production of dental enamel.
This is caused by exposure to high concentrations of fluoride when teeth are developing (between the ages of 3 months and 8 years).
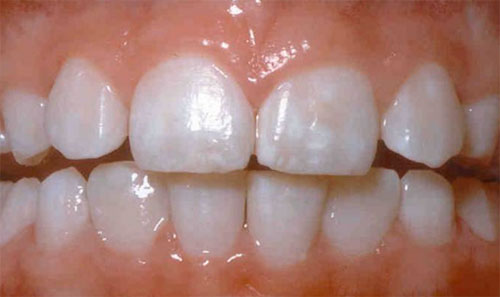
Signs of Fluoride Exposure
In its mild forms, fluorosis appears as tiny white streaks or specks in the tooth enamel. In its severe form, permanent pitting and brown discolorations can darken over time and disfigure the teeth.
As of 2005, 23% of persons in the United States aged 6 to 39 years had mild or more severe forms of dental fluorosis!
Apologies for the picture below – but this is what it looks like…
How Fluoride Causes Bone Loss
Fluorine activates both osteoblasts (bone-building cells) and osteoclasts (bone-resorbing cells). While fluoride may increase bone mass, the newly formed bone lacks normal structure and strength.
Under a microscope, the “crystallization pattern” of bone from fluoride-treated animals and humans proves abnormal.
In trabecular bone, the spongy interior portion of our bones, fluorine increases bone volume and thickness, but does not increase connectivity. This lack of trabecular connectivity reduces bone quality despite the increase in bone mass.
At very low and localized concentrations in dental implants, fluoride encourages osteoblast production and new bone formation. But at higher concentrations, fluoride blocks new bone formation.
In fact, high levels of fluorine in the body can cause skeletal fluorosis, a serious condition in which bones become hard and brittle, along with ligaments that can calcify, bone pain – and bone loss.
After ingestion, fluoride goes to the stomach where it reacts with stomach acid to form hydrogen fluoride. Hydrogen fluoride is absorbed from the gastrointestinal tract and sent into the portal vein, which delivers it to the liver.
In the liver, harmful compounds are usually transformed into something we can send out of the body in urine or bile. This biotransformation occurs through the activity of liver enzymes that first oxidize the harmful compound and then bind it to a carrier that deposits it in urine or bile for excretion.
Fluorine, however, is itself such a strong oxidizer– the strongest oxidizer currently known— that it easily blocks the liver’s attempts to prepare it for excretion and passes into the bloodstream, where it is rapidly distributed to all tissues, including our bones.
Once inside bones, fluorine attacks them through several mechanisms:
First, fluorine overwhelms bone cells’ ability to produce their most important antioxidant defender, glutathione. Once defenses have been eliminated, fluorine is free to wreak havoc, shut down osteoblasts and cause inflammation that increases osteoclast production and activity.
Next, fluorine accumulates in bone, binding to and shutting down alkaline phosphatase, an enzyme involved in the production of osteoblasts. Fluorine exposure also reduces levels of copper, zinc, manganese and other trace minerals required for bone building. The end result is that osteoblast production stops.
Our bones are largely composed of calcium compounds, up to 50% of which are hydroxyapatite. Fluorine converts hydroxyapatite to fluorapatite, which changes bones’ crystalline structure, delaying mineralization with calcium. This causes a reduction in bones’ mechanical strength properties.
Hydrogen fluoride reacts with calcium to form an insoluble salt, CaF2. This salt has to be cleared by the body, and as it goes, takes out some calcium from the bone matrix.
Fluorine also induces secretion of parathyroid hormone, which triggers the production of osteoclasts. Increased fluoride intake has been repeatedly shown to increase levels of parathyroid hormone circulating in the bloodstream and to cause hyperparathyroidism.
Parathyroid hormone signals osteoblast cells to secrete a signaling molecule called RANKL.
RANKL then summons the production of osteoclasts. For this reason, when our parathyroid hormone levels are chronically elevated, so is our production of osteoclasts – and we therefore lose bone.
The thing is, you may not realize the foods and beverages you’re consuming regularly contain significant amounts of fluoride. To discover common dietary sources and protect your health and bones, check out the table below.

Foods and Beverages with Highest Fluoride Content
Want to know which foods and beverages consumed in the U.S. have the highest fluoride content? Then check out the following table to see a brief list!
| Food | Micrograms of Fluoride in 100 grams (3 oz) |
|---|---|
| Wine, red | 105 |
| Wine, white | 202 |
| Carbonated water, fruit flavored | 105 |
| Coffee | 91 |
| Fruit juice drink, apple | 104 |
| Grape juice blend, (apple & grape) Juicy Juice | 102 |
| Grape juice, white | 204 |
| Tea, brewed, microwave | 322 |
| Tea, instant, powder, prepared with tap water | 335 |
| Tea, brewed, decaffeinated | 269 |
| Water, tap (Mid-west), municipal | 99 |
| Water, tap (Mid-west), well | 53 |
| Water, tap (Northeast), municipal | 74 |
| Water, tap (Northeast), well | 9 |
| Water, tap (South), municipal | 93 |
| Water, tap (South), well | 10 |
| Water, tap (West), municipal | 51 |
| Water, tap (West), well | 24 |
| Oatmeal, cooked | 72 |
| French fries, McDonald’s | 115 |
| Crab, canned | 210 |
| Shrimp, canned | 201 |
| Shrimp, fried | 166 |
| Fish sticks, baked | 134 |
The full listing of 400 foods across 23 food groups can be freely accessed at the National Fluoride Database.
Were you aware of all of the food and beverages that contain high fluoride levels listed above? Let me know in the comments below.
Protecting Your Bones From Fluoride
How can we protect ourselves from fluoride’s nasty effects in our bones?
Include calcium and magnesium-rich foods in your diet! Calcium and magnesium ions form insoluble complexes with fluoride, reducing its absorption and increasing its elimination in stools.
Eat plenty of fruits and vegetables. A high intake of plant foods increases the pH of your urine (makes it more alkaline), which increases the kidneys’ ability to excrete fluoride.
And take AlgaeCal Plus, which delivers both plant-based calcium and magnesium, further lessening fluorine’s bioavailability.
Avoid fluoride-containing toothpaste by checking the ingredient list on your tube. It’s not always clear that toothpaste contain fluoride.
In fact, fluoride may be listed as:
- Sodium fluoride (NaF)
- Stannous fluoride (SnF2)
- Olaflur (an organic salt of fluoride)
- Sodium monofluorophosphate (Na2PO3F)
Most toothpaste sold in the United States contains 1,000 to 1,100 parts per million (ppm) fluoride. In the UK, the fluoride content is often higher; a 1,450 ppm fluoride is not uncommon.
According to the Institute of Medicine (IOM) guidelines in 1997, fluoride intakes of 0.01 mg/day for infants through 6 months, 0.05 mg/kg/day (meaning milligram per kilogram of body weight per day) beyond 6 months of age, and 3 mg/day and 4 mg/day for adult women and men (respectively), are adequate to prevent cavities.
IOM set upper limits for fluoride at 0.10 mg/kg/day in children less than 8 years and 10 mg/day for those older than 8 years.
But consumption of even the lowest amount of fluoride recommended by the IOM to prevent cavities is unnecessary.
Why? Xylitol – a natural sugar found in trace amounts in berries and vegetables- is a great, safe alternative to fluoride. In fact, AlgaeCal chose to use xylitol as its natural sweetener for Triple Power Omega 3 Fish Oil because of its beneficial properties and safety.
Xylitol not only prevents plaque formation and cavities, but is shown to even reverse developing cavities and restore healthy tooth enamel — safely.
Our bodies already produce xylitol in tiny amounts, but can benefit from more.¹
Studies demonstrate that 4 to 12 grams of xylitol per day is safe and effective.
The easiest and best way to deliver xylitol to your teeth?
Xylitol-containing chewing gums, which keep the xylitol in contact with teeth far longer than toothpaste or mouthwash. If a piece of gum contains 1 gram of xylitol, then cavity protection, and minty fresh breath, can be easily achieved by chewing 4 or more pieces throughout the day.
The Bottom Line on Fluoride
Chronic exposure to too much fluoride is harmful to your bones.
And when you realize that chronic exposure to too much fluoride is the status quo in our everyday lives, minimizing your exposure becomes an obvious part of your healthy bones’ game plan.
Avoiding fluoride altogether is impractical. Hey, I’m not giving up a glass of Merlot with dinner several times a week! But I am suggesting that you:
- Choose a fluoride-free toothpaste
- Consider your water source
- Brew your tea for the shortest amount of time you find delivers the taste you prefer (the longer tea sits, the more fluoride will appear in your cup)
- Make foods rich in calcium and magnesium staples in your diet and take your AlgaeCal Plus to reduce fluoride’s effects.
- And don’t forget about xylitol– a natural, healthy alternative to fluoride that will protect your teeth without harming your bones!
What changes are you going to make – or have already – to reduce your fluoride intake? I’m curious to hear your thoughts!
Book Source:
- Wright JV. Fight tooth decay and dramatically slash or even eliminate dental cavities for a lifetime! Nutrition & Healing. Vol. 19, Issue 3, May 2012.




Laura Ricci
August 19, 2017 , 6:52 amThe link to the food list has been removed. Is there another source? I have no idea where fluoride might come from from this article.